
资料发布时间:2018-10-30 14:50:00 最后更新时间: 2021-06-09 15:17:08
【药品简介】 达卡他韦(Daklinza)是一种丙型肝炎病毒NS5A抑制剂适用为与索非布韦使用为慢性HCV基因型3感染的治疗。口服,每天1次与索非布韦联用共12周
【适应症状】 达卡他韦,达卡他韦是一种丙型肝炎病毒(HCV)NS5A抑制剂适用为与索非布韦使用为慢性HCV基因型3感染的治疗
【 药品别名 】 达卡他韦 Daclatasvir Daklinza
印度达卡他韦(达卡他韦 Daclatasvir Daklinza)价格,正品图片,治疗丙肝药物, 购买达卡他韦请到印度药房官方网站进行购买 【印度药房大全目录】
【 市场参考价格 】 ¥178.00~405.00
播放/暂停

生物活性
|
产品描述 |
Daclatasvir (BMS-790052) is a highly selective inhibitor of HCV NS5A with EC50 of 9-50 pM, for a broad range of HCV replicon genotypes and the JFH-1 genotype 2a infectious virus in cell culture. Phase 3. |
|
靶点活性 |
HCV NS5A,9pM-50pM(EC50) |
|
体外活性 |
BMS-790052 is one of the most potent inhibitors of HCV replication reported so far. The mean EC50 valuses of BMS-790052 are 50 and 9 pM for HCV genotype 1a and 1b replicons, respectively. BMS-790052 displays a therapeutic index (CC50/EC50) of at least 105 and is inactive towards a panel of 10 RNA and DNA viruses, with EC50 higher than 10 μM. This confirms BMS-790052's specificity for HCV. [1] In Huh7 cells harboring the HCV genotype 1b replicons, BMS-790052 blocks both transient and stable HCV genome replication, with EC50 values raging from 1-15 pM. BMS-790052 (100 pM or 1 nM) has been shown to alter the subcellular localization and biochemical fractionation of NS5A. [2] BMS-790052 inhibits hybrid replicons containing HCV genotype-4 NS5A genes with EC50 of 7-13 pM. Residue 30 of NS5A is an important site for BMS-790052-mediated resistance in the hybrid replicons. [3] |
|
体内活性 |
In a randomized, double-blind, placebo-controlled, single ascending-dose study, Daclatasvir (BMS-790052) is administered at six dose levels to healthy, non-HCV-infected subjects over a range of 1 to 200 mg as an oral solution. Daclatasvir is safe and well tolerated up to 200 mg with no clinically relevant adverse effects. After oral administration, Daclatasvir is readily absorbed, with dose-proportional exposures over the studied dose range, and all subjects have drug concentrations greater than the protein-binding-adjusted EC90 for genotypes 1a and 1b, as measured in the replicon assay, at and beyond 24 h post-dose. (The protein binding-adjusted EC90 figures are derived from an analysis of the effect of the addition of human serum on antiviral activity in replicons. In the presence of 40% human serum, the EC90 for Daclatasvir is 383 pM (0.28 ng/mL) for the genotype 1a replicon and 49 pM (0.04 ng/mL) for the genotyope 1b replicon)[1]. Mice in each group that developed persistent HCV infection are divided into two treatment groups. One group receive 4 weeks of Asunaprevir/Daclatasvir treatment and the other group received 4 weeks of Ledipasvir/GS-558093 treatment. Asunaprevir/Daclatasvir therapy and Ledipasvir/GS-558093 therapy rapidly decease serum HCV RNA levels to below the sensitivity, and they are not detected after completion of the therapy except for two mice in the Ledipasvir/GS-558093 group[5]. |
|
激酶实验 |
FRET assay for HCV NS5A inhibitors: The peptide (Ac-Asp-Glu-Asp [EDANS]-Glu-Glu-Abu-[COO] Ala-Ser-Lys [DABCYL]-NH2) contains a fluorescence donor {EDANS, 5-[(2-aminoethyl)amino]naphthalene-1-sulfonic acid} near one end of the peptide and an acceptor {DABCYL, 4-[(4-dimethylamino)phenyl]azo)benzoic acid} near the other end. Intermolecular resonance energy transfer between the donor and the acceptor quenches the fluorescence of the peptide, but as the NS3 protease cleaves the peptide, the products are released from resonance energy transfer quenching. The fluorescence of the donor increases over time as more substrate is cleaved by the NS3 protease. The assay reagent is: 5× luciferase cell culture lysis reagent diluted to 1× with dWater, NaCl (150 mM), the FRET peptide (20 μM). HCV-Huh-7 cells are placed in a 96-well plate, and allowed to attach overnight (1×104 cells per well). The next day, BMS-790052 is added to the wells and the plate is incubated for 72 hours. The plate is then rinsed with PBS and used for the FRET assay by the addition of 30 μL of the FRET peptide assay reagent (described above) per well. Signals are obtained using the Cytofluor 4000 instrument, which has been set to 340 nm (excitation)/490 nm (emission) automatic mode, for 20 cycles or less, with the plate being read in the kinetic mode. Following FRET, 40 μL of luciferase substrate is added to each well and the luciferase is measured. |
|
细胞实验 |
BMS-790052 is added to 96-well plates containing HCV replicon cells seeded approximately 12 hours before in 200 µL media.The cell plates are tested for replication activity and cytotoxicity after 72 hours of incubation. Cytotoxicity is measured with CellTiter-Blue, after which the media and dye are removed, plates are inverted and the remaining liquid is blotted with paper towels. Replication activity of the HCV genotype 1a cell lines is quantified using Renilla luciferase. 1× Renilla luciferase lysis buffer (30 µL) is added to each well and plates are incubated with gentle shaking for 15 min. Renilla luciferase substrate (40 µL) is then added and the signals are detected using a Top Count luminometer set for light emission quantification. One hundred per cent activity is calculated for each cell line for the DMSO-only wells; percentage activity is calculated for each concentration of the inhibitor by dividing the average value for wells containing compound by the average value for wells containing DMSO.(Only for Reference) 细胞系: HCV replicon cells (Huh7) |
|
动物实验 |
动物模型:Mice |
化学信息
|
分子量 |
738.88 |
|
分子式 |
C40H50N8O6 |
|
CAS |
1009119-64-5 |
|
溶解度 |
DMSO: 136 mg/mL (184.1 mM) Ethanol: 136 mg/mL (184.1 mM) Water: <1 mg/mL ( < 1 mg/ml refers to the product slightly soluble or insoluble ) |
|
储存条件 |
store at -80°C |
|
备注 |
For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. |
配制溶液
| 1 mg | 5 mg | 10 mg | |
| 1 mM | 1.353 ml | 6.767 ml | 13.534 ml |
| 5 mM | 0.271 ml | 1.353 ml | 2.707 ml |
| 10 mM | 0.135 ml | 0.677 ml | 1.353 ml |
| 50 mM | 0.027 ml | 0.135 ml | 0.271 ml |

丙肝患者知道目前最权威的吉三代是对6种丙肝基因型达到通杀的疗效,治愈率约为99%。它可以使丙型肝炎患者完全治愈,它是最受......

吉三代是治疗丙型肝炎的特效药,是目前治疗丙型肝炎最好的药物。那么用吉三代治疗丙肝具有什么优势呢? 吉三代的优势主要分为以......

服用达卡他韦的注意事项: 1、每天按时服用达卡他韦,不要错过,每天同时服用。 2、早晚吃都可以。一般来说,最好在饭前或饭......
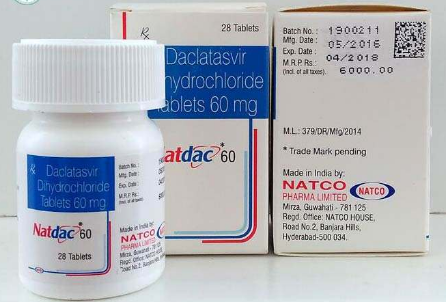
索非布韦与达卡他韦都是治疗丙肝的药物,可以直接口服。索菲布韦是NS/5B核苷聚合酶抑制剂,达卡他韦是NS/5A非核苷聚合......
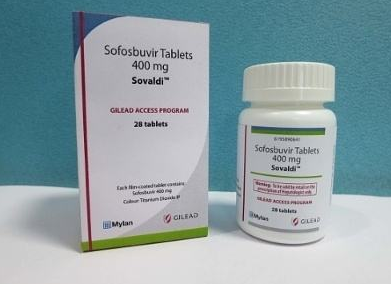
索非布韦它是美国吉利德公司的第一种丙型肝炎抗病毒治疗药物,俗称的吉一代,但在今天仍然是过时的,它仍然是我们治疗丙型肝炎的......

丙肝治疗的药物,干扰素是通过它有一个免疫调节的作用来治疗丙肝...
没法来说甲肝、乙肝、丙肝哪一个严重,因为还要根据发病的具体的情况来定...

北京华大中医医院讲解:丙肝患者怎么做才能生育健康宝宝。...

在治疗前应明确患者的肝脏疾病,是否由丙型肝炎病毒感染引起...
Pharmacy Catalogue
【代购】【有实体店】【在线客服懂中文】
代购+直邮,印度抗癌药跨境医药电商直邮服务,让肿瘤患者能够有更好的选择,平台提供印度就医,印度易瑞沙,吉三代,吉二代,索......
【禁止中国访问 】【印度连锁】【品牌实力】
MedPlus是印度第二大药房连锁店。它经营1555家商店,其中1317家为公司所有,159家为特许经营公司,43家为医......
【药品种类丰富 】【渠道正规】【价格较高】
Wellness Forever是一家生活方式零售药房连锁店,致力于为印度本土客户提供全面的健康服务。目前总收入约为62......
【visa/万事达】 【全球可邮 】【纯线上销售】【可开处方】
Netmeds.com致力于开发印度本土市场,已成为印度领先的在线药店。Netmeds.com通过其全天候在线门户和移动......